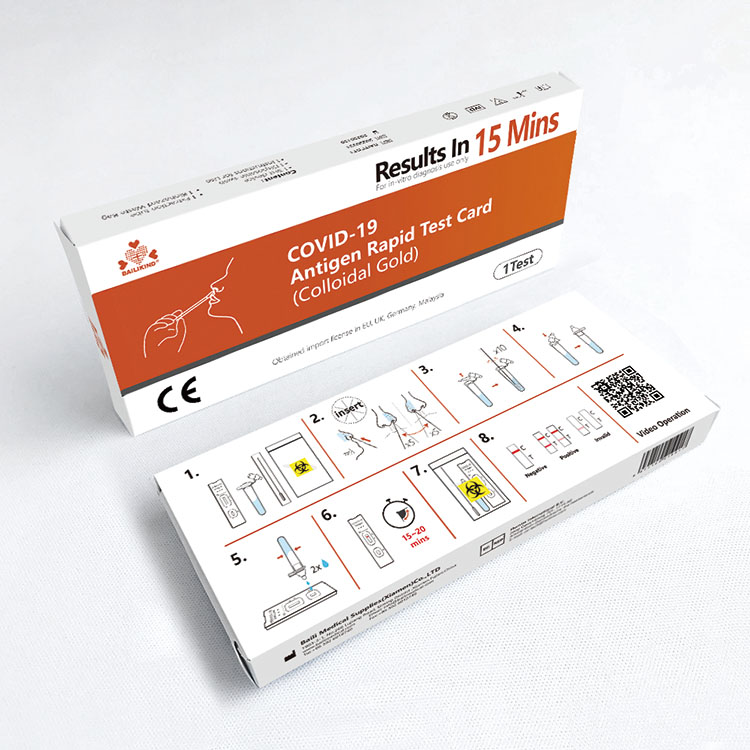
COVID-19 Antigen Rapid Test Card Colloidal Gold
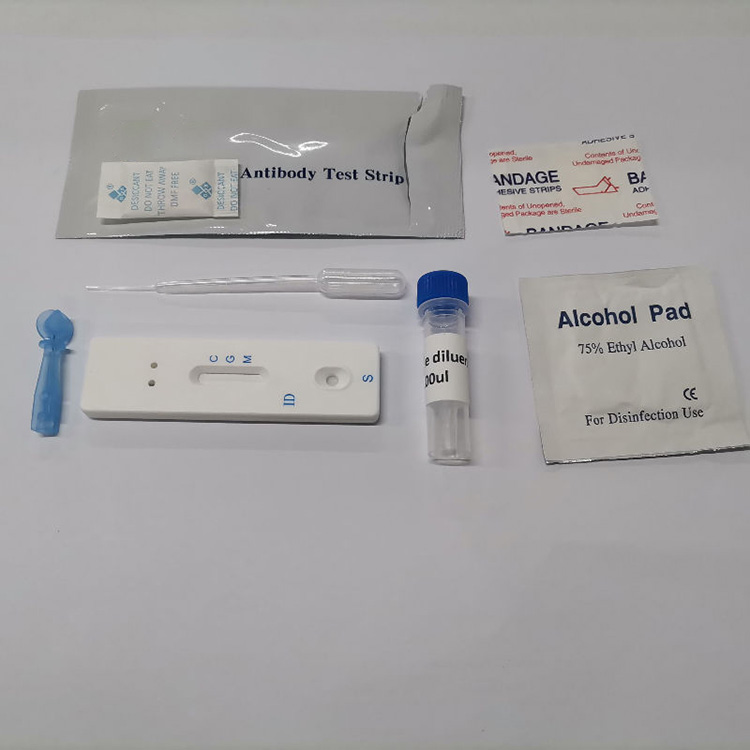
COVID-19 Antigen Rapid Test Card Colloidal Gold is intended for the qualitative detection of SARS-CoV-2 Antigen (Nucleocapsid protein) in human saliva samples in vitro.
Send Inquiry
PDF DownLoad
1. Product Introduction of COVID-19 Antigen Rapid Test Card Colloidal Gold
COVID-19 Antigen Rapid Test Card (Colloidal Gold) is intended for the qualitative detection of SARS-CoV-2 Antigen (Nucleocapsid protein) in human saliva samples in vitro.
Which are applied to the following scenarios:
1. mass population screening,such as hospital, airport, station, community, etc.
2. short-term continuous surveillance.

2. Product Features of COVID-19 Antigen Rapid Test Card (Colloidal Gold)
* Anterior nasal swab specimen, noninvasive
* Very simple to use
* Convenient, no instrument required
* Rapid, results within 15~20 minutes
* Cost-efficient

3. Product Details of COVID-19 Antigen Rapid Test Card (Colloidal Gold)











4. How to detect SARS-CoV-2 virus?
The SARS-CoV-2 virus particle is made up of five components: an RNA gene chain and four proteins. The outermost layer of the particle is a Spike Protein (S), and the viral Envelope below the Spike is composed of Envelope Protein (E) and Membrane Protein (M). The core contained within the envelope is a helical folded structure composed of RNA gene chains and Nucleocapsid proteins (N). Using the principle of specific binding of antigen and antibody, the presence of SARS-CoV-2 antigen(Nucleocapsid protein) can be detected by antibody.

Advantages over Elisa and PCR
| Method | ELISA Kit | RT-PCR | Colloidal Gold Test Kit (Colloidal Gold) |
| Instrument Cost | Expensive | Expensive | Cheap |
| Detection Time | Longer | Longer | Short |
|
Amplification Specificity |
Stronger | Stronger | Stronger |
|
Environmental Requirements |
High | High | Low |
|
Operation Difficulty |
High | High | Low |
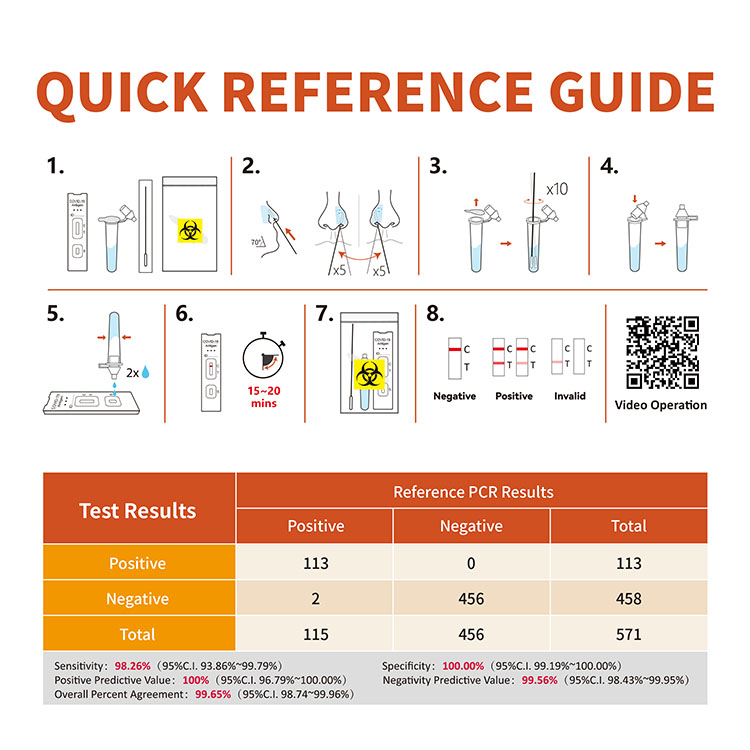
The performance of the SARS-CoV-2 Antigen Rapid Test (Colloidal Gold) was established with 859 specimens prospectively collected from individual symptomatic patients who were suspected of COVID-19. Anterior nasal swab specimens were collected and tested according to the requirements of the Instructions for Use. The storage, transportation and detection of samples after collection met the relevant requirements of the Instructions for Use. At the same time, SARS-CoV-2 was detected by emergency nucleic acid detection reagent.
Clinical performance summary of the WIZ’S SARS-CoV-2 Antigen Rapid Test (Colloidal Gold)
| Test Results | Reference PCR Results | ||
| Positive | Negative | Total | |
| Positive | 328 | 0 | 328 |
| Negative | 14 | 517 | 531 |
| Total | 342 | 517 | 859 |
PPA: 95.91% (C.I. 93.25%~97.55%)
NPA: 100.00% (C.I. 99.26%~100.00%)
OPA: 98.37% (C.I. 97.28%~99.03%)
We, the manufacturer, herewith, declares that the product(s) as specified above meet(s) the applicable provisions of the European Directive 98/79/EC on in vitro Diagnostic Medical Devices. All supporting technical documentation demonstrating compliance is kept by the manufacturer and can be made available by the Authorized Representative in Europe.

5. Product Packing of COVID-19 Antigen Rapid Test Card (Colloidal Gold)
Company Certification

Company Profile


Company Exhibition


6. Deliver,Shipping and Serving Of COVID-19 Antigen Rapid Test Card (Colloidal Gold)
| Shipping Method | Shipping Terms | Area |
| Express | TNT /FEDEX /DHL/ UPS | All Countries |
| Sea | FOB/ CIF /CFR /DDU | All Countries |
| Railway | DDP | Europe Countries |
| Ocean +Express | DDP | Europe Countries /USA/Canada/Australia /Southeast Asia /Middle East |
7. FAQ of COVID-19 Antigen Rapid Test Card (Colloidal Gold)
A:Both.We have been in this field for more than 7years.With superior quality products and competitive price,we sincerely hope to develop mutual-beneficial business with our customers all over the world.
A: T/T,L/C,D/A,D/P and so on.
A: EXW, FOB, CFR, CIF, DDU and so on.
A: Normally, it will take 15 to 30 days after receiving the deposit The specific delivery time depends on the items and the quantity of your order.
A: Yes, we can produce by your samples or technical drawings.
A: If the quantity is small, the samples will be free, but the customers have to pay the courier cost.
A: Yes, we have 100% test before delivery.
A: We keep good quality and competitive price to ensure our customers benefit ; and we respect every customer as our friend and we sincerely do business and make friends with them.